SES Power explains the principles, materials and prospects of sodium-ion batteries for you
Na-ion batteries first appeared in the early 1980s, and then due to the better performance of lithium-ion batteries, the research on sodium-ion batteries was stagnant. After 2010, with the increasing demand for lithium batteries in various industries around the world, the materials for lithium-ion batteries are in short supply, and people's research on sodium-ion batteries has re-emerged.
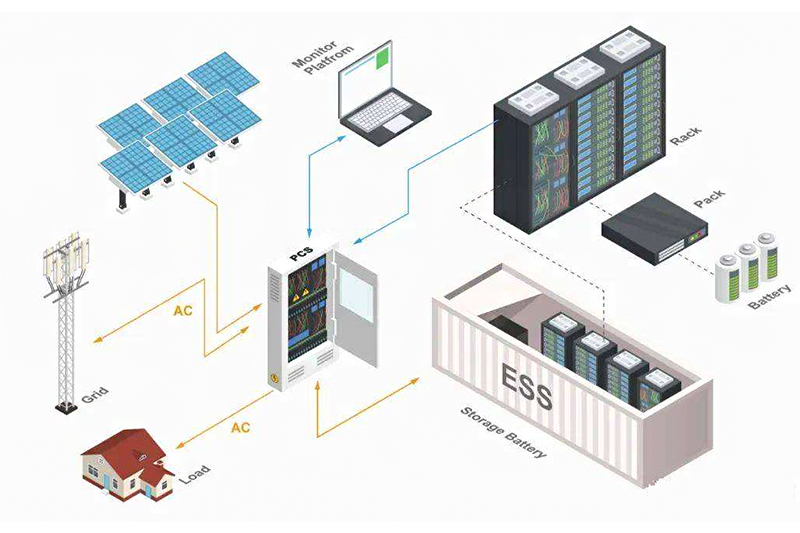
SES Power often uses lithium batteries to customize products, such as 12V100Ah, 24V100Ah, 36V100Ah, 48V100Ah using EVE, CATL, BYD square aluminum lithium iron phosphate batteries, home energy storage 3KW, 5KW systems, rack-mounted energy storage systems and other products. But we are quite concerned about the development of sodium-ion batteries because it means endless possibilities. Let the senior engineers of SES Power analyze the principle, composition and prospects of sodium-ion batteries for you.
The working principle of sodium ion batteries mainly relies on the movement of sodium ions between the positive electrode and the negative electrode to work: during charging, sodium ions are deintercalated from the positive electrode, and swim through the diaphragm in the electrolyte to embed the negative electrode, and the negative electrode is in a sodium-rich state; On the contrary.
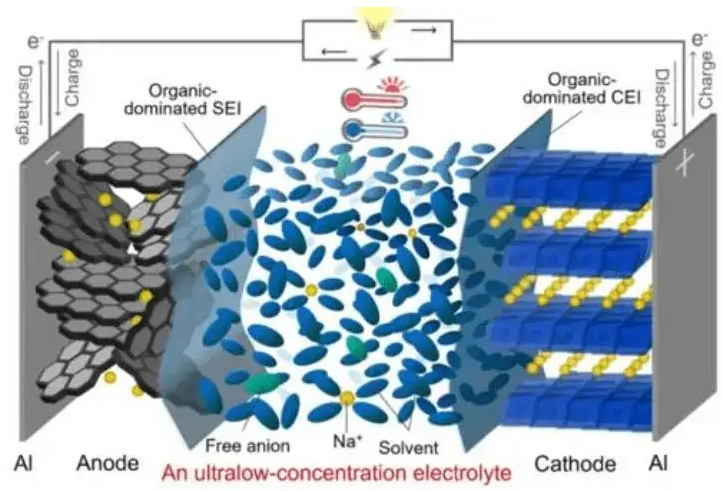
(Schematic diagram and internal structure of sodium-ion battery)
The advantages of sodium-ion batteries are relatively high energy density, high safety in use (can be discharged to 0V), environmental protection (without lead, cadmium, mercury and other elements that pollute the environment), wide resource sources, and low cost.
Due to the working principle of sodium-ion batteries and the high similarity of battery materials to lithium-ion batteries, the process and equipment for battery production can be reused. This also means that the investment in battery factories will be greatly reduced.
According to different application scenarios, sodium-ion batteries can be mainly divided into power and energy storage. Compared with the mature commercialization level of lithium-ion batteries, the commercialization of sodium-ion batteries is only in its infancy. Only a few companies have carried out preliminary commercialization of sodium-ion batteries, and a complete and mature industrial chain has not yet formed.
Because sodium-ion batteries work similarly to lithium-ion batteries, many materials and processes can be reused, even from a raw material perspective. In terms of raw materials, the first and most obvious difference between sodium-ion batteries and lithium-ion batteries is the difference in working ions.
A: Positive electrode material
At present, the cathode materials for sodium-ion batteries are synthesized in the laboratory, and the sources of sodium are very wide, but large-scale industrial production has certain requirements for cost, process safety, acidity and alkalinity, and carbonic acid is most likely to become the raw material for industrial production. Sodium (i.e. soda ash).
Contrary to the uneven and scarce global distribution of lithium resources, sodium resources are widely distributed around the world, and its abundance in the earth's crust ranks sixth. The salt we eat daily is the most common sodium salt - sodium chloride. The most common sources of sodium chloride are sea salt, lake salt, and rock salt (mineral salt).
The structure of sodium ion battery mainly includes five parts: positive electrode material, negative electrode material, electrolyte, current collector and separator. Cathode Materials Cathode and anode materials affect key performance indicators such as energy density, power density, cycle life, and safety of Na-ion batteries, and are critical to battery performance.
Different from the situation in which the cathode technology route of lithium-ion batteries is basically determined, there are currently more than 100 cathode materials related to sodium-ion batteries, and the technological route is still in the process of evolution. According to the composition, mainstream sodium-ion battery cathode materials can be divided into layered metal oxides, polyanionic compounds and Prussian blue compound systems.
B: negative electrode material
The negative electrode material is different from the graphite material negative electrode used in lithium ion batteries. Because the graphite layer spacing is too small, the intercalation of sodium ions with a larger radius between the graphite layers requires more energy, and reversible deintercalation cannot be performed within the effective potential window. Therefore, it is considered that Conventional graphite cannot be used as a negative electrode for sodium-ion batteries.
The research directions of anode materials for sodium ion batteries include hard carbon, soft carbon, titanium-based oxides and alloys, etc. The research on hard carbon is the most, and the current commercial sodium ion batteries also use hard carbon materials as negative electrodes.
C: Diaphragm
The separator is used to isolate the positive and negative electrodes of sodium-ion batteries, preventing short-circuit phenomena, and at the same time acting as an ion channel.
At present, the electrolyte of the most common organic electrolyte-based sodium-ion battery is very similar to that of the lithium-ion battery, and the solvent remains unchanged, except that the solute salt is changed from lithium hexafluorophosphate to sodium hexafluorophosphate, so it can be used with lithium ion Battery-like separator.
D: Electrolyte
The electrolyte is the ionic charge carrier necessary for the electrochemical reaction and is the key to improving the power characteristics of Na-ion batteries. Electrolytes are mainly composed of solvents, solutions, additives and the like.
The most common electrolyte for sodium-ion batteries is an organic liquid electrolyte. The organic solvent portion of the organic electrolyte is similar to the corresponding composition of lithium-ion batteries.
E: positive and negative carriers
Unlike lithium, since aluminum and sodium do not undergo alloying reactions at low potentials, the carriers of the positive and negative electrodes of sodium-ion batteries, that is, current collectors, can use inexpensive aluminum foils instead of higher-cost copper foils. The current collector aluminum foil of sodium ion battery is basically the same as that of lithium battery, and the performance requirements are basically similar.
Since the commercialization level of sodium-ion batteries is only in its infancy, there are relatively few studies on battery systems. CATL has developed the AB battery system solution in terms of battery system integration, that is, the sodium-ion battery and lithium-ion battery are mixed and matched in a certain proportion, integrated into the same battery system, and the balance control of different battery systems is carried out through the BMS accurate algorithm.
Application market in the current secondary battery market, lithium-ion batteries are the absolute main force and core. Although lithium-ion batteries are relatively free of obvious performance limitations, sourcing raw materials such as lithium carbonate and lithium cobaltate is becoming increasingly difficult.
SES Power believes that although the original intention of sodium-ion battery research and development is to supplement and replace the application of lithium-ion batteries in the field of power batteries, because the current energy density of sodium-ion batteries is not as good as that of lithium-ion batteries, it is temporarily unable to shake the lithium-ion battery-led electric power automotive field. In terms of stationary energy storage, sodium-ion batteries have broad prospects due to their low requirements for volume and quality. However, from the public information, the cycle life of sodium-ion batteries is much lower than that of lithium iron phosphate batteries, and there is no great advantage in cost, especially when lithium iron phosphate batteries replace lead-acid batteries, even if sodium-ion batteries can officially mass production can not replace lithium iron phosphate batteries for a long time.
As for our lithium iron phosphate battery that can be used in an environment of -40 ℃, it has been stabilized after years of research and development and experiments. We believe that sodium-ion batteries are even more irreplaceable.